Formerly used as a refrigerant and as an aerosol propellant.
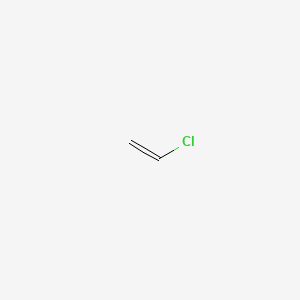
Vinyl chloride lewis structure.
The metabolic capacity of rats is saturated at atmospheric concentrations of 250 ppm vinyl chloride and 55 ppm vinyl bromide.
A step by step explanation of how to draw the c2h3cl lewis dot structure.
It has a pungent odor of garlic or onions.
Write a lewis structure for each of the following organic molecules.
Structure properties spectra suppliers and links for.
For the c2h3cl lewis structure calculate the total number of valence electrons for.
Acrylonitrile is an organic compound with the formula ch 2 chcn.
All three fluorines are bonded to the same carbon c c 2 cl 2 f 4 freon 114.
The electron configuration of carbon is 1s 2 2s 2 2p 2.
A nonflammable inhalation anesthetic.
It is a colorless volatile liquid although commercial samples can be yellow due to impurities.
The atomic number of carbon atom is 6.
The valence electrons are 4.
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition.
Vinyl chloride is a mutagen having clastogenic effects which affect lymphocyte chromosomal structure.
Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics.
Here the three atoms are carbon hydrogen and chlorine.
The vinyl chloride molecule has 18 valence electrons 4 from each c atom 1 from each h atom and 7 from cl all of which are accounted for by the above lewis structure.
Here the unshared pair of electrons is to be shown.
The easiest way to determine formal charge for an atom is to compare its valence electrons with the number of electrons it gets in a molecule.
So here the valence shell is the second shell.
In terms of its molecular structure it consists of a vinyl group linked to a nitrile it is an important monomer for the manufacture of useful plastics such as polyacrylonitrile.
Vinyl chloride is a group 1 human carcinogen posing elevated risks of rare angiosarcoma brain and lung tumors and malignant haematopoeitic lymphatic tumors.
For all the halogenated ethylenes studied so far including vinyl chloride and vinyl bromide metabolism in vivo is a dose dependent saturable process.