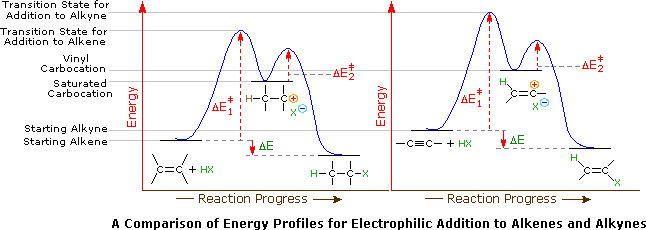
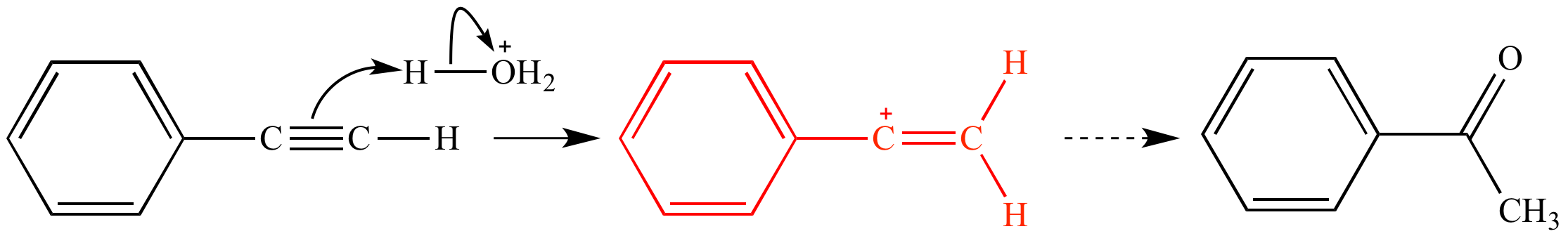
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.
Vinylic carbocation stability.
Carbocation stability resonance rearrangement allylic vinylic examples organic chemistry duration.
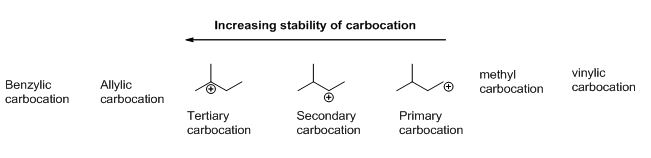
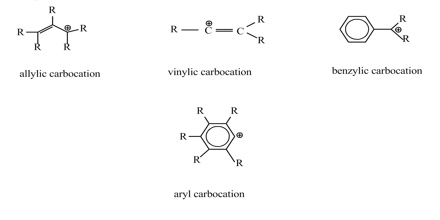
An allylic carbocation in which an allylic carbon bears the positive charge.
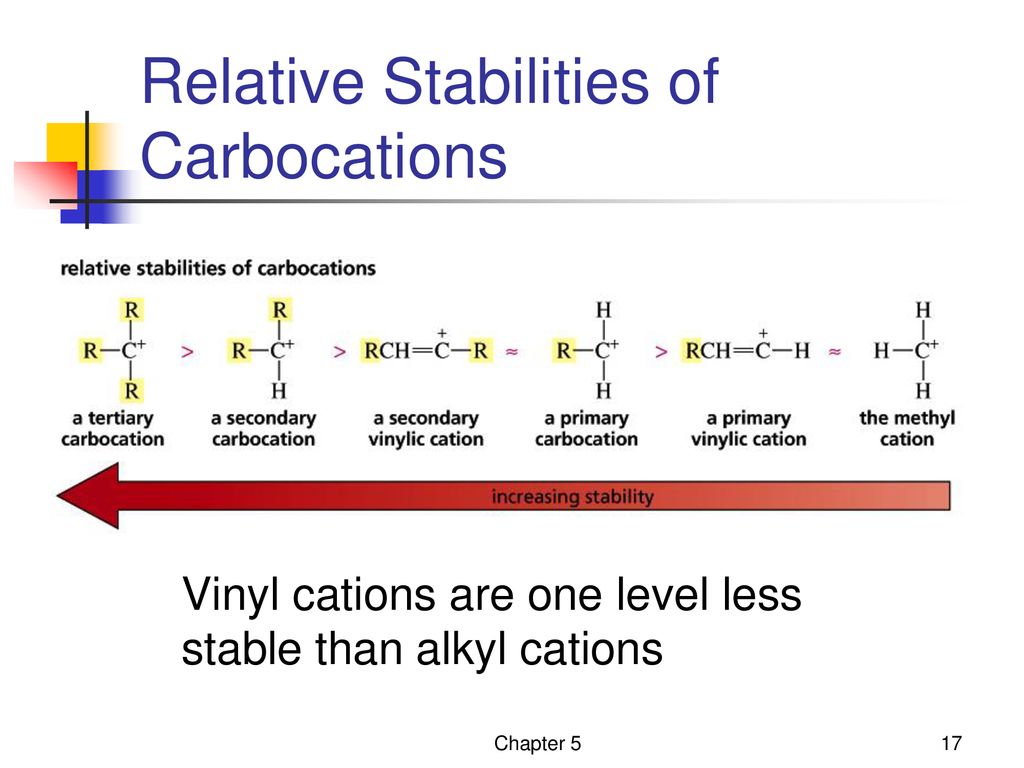
Due to this reason the stability of a primary vinylic carbocation is less than a primary alkyl carbocation.
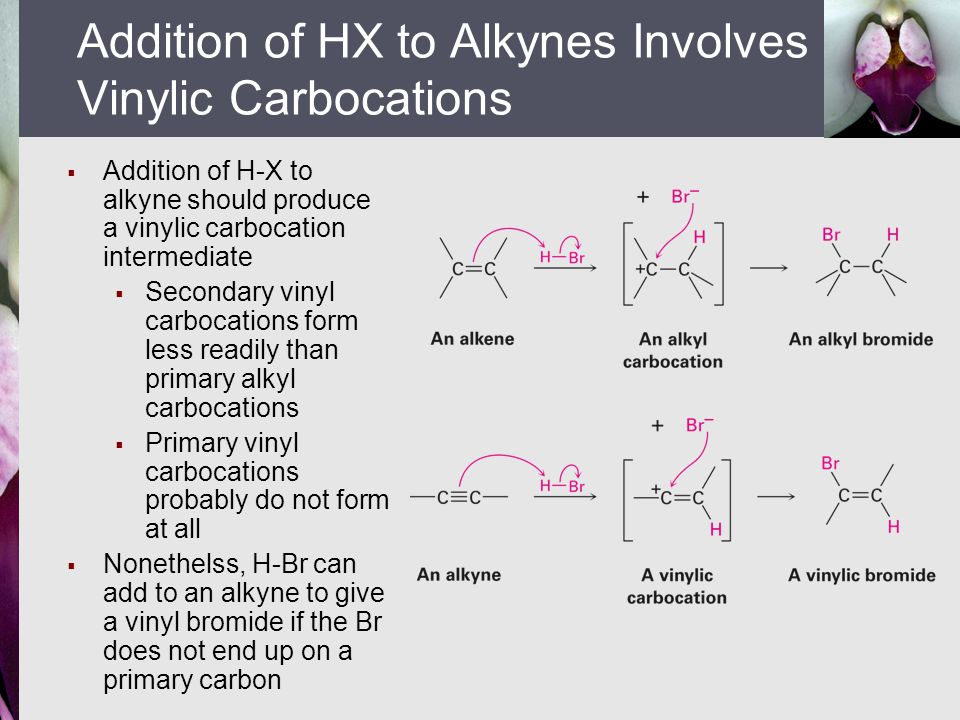
A vinylic carbocation is a positively charged carbon that is attached to a non carbon and participates in a double bond with another carbon.
An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
Stability of carbocation intermediates.
Table of contents formation of the carbocation carbocations carbocation stability carbocations often occur as intermediates in reactions in organic chemistry.
The organic chemistry tutor 100 569 views 15 37.
Vinyl and aryl carbocations are very rare to find due to their low stability.
It is therefore important to get acquainted with its characteristics.
In the same way lower stability of aryl carbocation in comparison to a secondary alkyl carbocation can be explained.
Atoms or groups attached to an allylic carbon are termed allylic substituents.
This will help you master carbocation intermediate reactions down the line including markovnikov alkene addition reactions unimolecular substitution sn1 β.
The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation forms.
We know that the rate limiting step of an s n 1 reaction is the first step formation of the this carbocation intermediate.
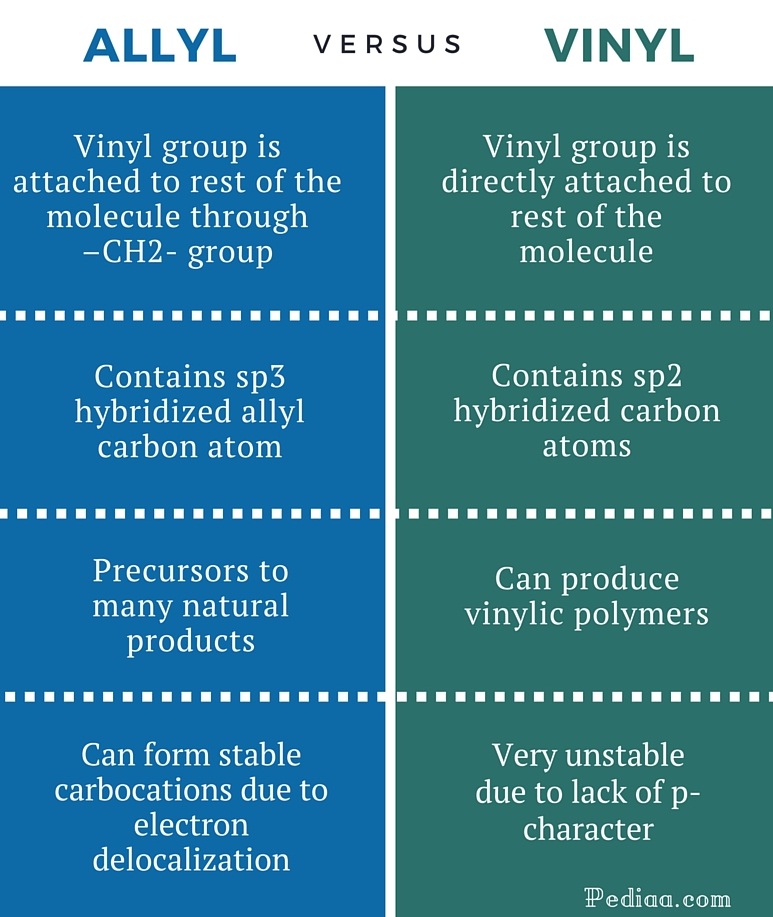
Do not confuse an allylic group with a vinyl group.
A vinyl carbocation has a positive charge on the same carbon as the double bond.
Vinylic carbocations are not able to undergo resonance.
It provides plenty of examples including allylic and vinyli.
Allylic carbocations are able to share their burden of charge with a nearby group through resonance.